BACKGROUND
Richter transformation (RT), most commonly manifesting as diffuse large B-cell lymphoma (RT-DLBCL), occurs in 2-8% of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). Patients who develop RT-DLBCL have a median overall survival (OS) of less than 12 months despite intensive chemoimmunotherapy. Immune checkpoint inhibitors (PD1 inhibitors) have shown clinical activity in RT-DLBCL, thus providing for a novel therapeutic approach. Such studies have shown a response to PD1 blockade associated with an immune evasion phenotype based on PD1 and/or PD-L1 expression on tumor cells, but the status of predictive biomarkers of response to PD1 blockade in RT-DLBCL remains largely unknown. Other lymphomas associated with PD-L1 expression often express CD30 and harbor Epstein-Barr virus (EBV), both presenting additional possible therapeutic targets, but few studies have assessed the frequency of these markers in RT-DLBCL.
METHODS
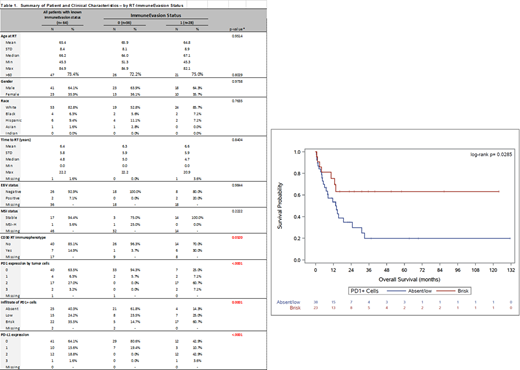
The study group consists of 64 patients with extramedullary biopsy confirmed RT-DLBCL. Expression of PD1, PD-L1, CD30, and microsatellite instability (MSI) status (hMLH1, hMSH2, hMSH6, PMS1) was assessed using immunohistochemistry on formalin-fixed paraffin-embedded pre-treatment tumor samples. EBV-encoded RNA was evaluated using colorimetric in situ hybridization. PD1 and PD-L1 expression levels were categorized on the basis of tumor cell expression as follows: negative (<5%), low-positive (5-50%), or high-positive (>50%). An "immune evasion phenotype" (IEP) was defined as RT-DLBCL cases having high-positive expression of PD1 and/or PD-L1 on tumor cells. The level of PD1-positive tumor-infiltrating lymphocytes (TILs) was estimated as a fraction of total lymphocytes and categorized as negative/low vs. brisk (>20%). Demographic and clinical features by IEP status were compared using chi-square/Fisher's exact tests for categorical variables and t-tests/Wilcoxon rank-sum tests for continuous variables. Kaplan-Meier analysis and corresponding log-rank tests were used to estimate and compare OS between groups defined by clinical features.
RESULTS
Patients included 41 men (64.1%) and 23 women with a median age of 66.2 years (range, 45.3-84.9 years). The interval from initial CLL/SLL diagnosis to RT-DLBCL was 4.8 years. PD-L1 expression on tumor cells was high-positive in 13 (20.3%), low-positive in 10 (15.6%), and negative in 41 (64.1%) cases. PD1 expression on tumor cells was high-positive in 19 (30.2%), low-positive in 4 (6.3%), and negative in 40 (63.5%) cases. Accordingly, 28/64 (43.7%) patients had IEP+ RT-DLBCL. A brisk level of PD1+ TILs was significantly more common in IEP1+ compared with IEP- tumors (17/28, 60.7% vs. 5/34, 14.7%; p=0.001). In addition, CD30 expression was significantly more common in IEP+ compared with IEP- RT-DLBCL (14/20, 70% vs. 1/27, 3.7%; p=0.0320). Two (2/36; 5.5%) cases were positive for EBV, both IEP+. There was no significant difference between the two groups in terms of age, sex, or time to transformation. Assessment of mismatch repair proteins demonstrated an MSI-stable phenotype in all but one (1/18; 5.5%) case; this case was MSI-high, IEP-, and had a brisk infiltrate of PD1+ TILs. Patients had a median OS of 13.6 months (95% CI: 10.0-19.5 months). Notably, patients with brisk PD1+ TILs had a significantly better OS compared to those with a negative/low infiltrate (p=0.0285).
CONCLUSIONS
We demonstrate that IEP+ status is common in RT-DLBCL and correlates with CD30 expression and a brisk infiltrate of PD1+ TILs. Our data also suggest that IEP+ status correlates with more favorable OS in RT-DLBCL.
Jain:Incyte: Research Funding; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; BMS: Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Aprea Therapeutics: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.